Modeling neurological disorders
RESEARCH

Our research is for modeling neurological disorders using reconstitution and stem cell-based approach with a focus on vesicle fusion and calcium signaling.
1) Modeling neurodevelopmental disorders using iPSC-derived neurons and brain organoids.
2) Extracellular vesicle (EV) biomarkers and EV-mediated inflammation in neurological disorders.
3) Lipid dysregulation in neurodegenerative diseases.
4) New paradigm of RIBOMONE in physiology and pathology.

Although microRNA (miRNA) regulates gene expression inside the cell where they are transcribed, extracellular miRNA has been recently discovered outside the cells, proposing that miRNA might be released to participate in cell-to-cell communication (Front Endocrinol. 2017).
My group first reported the active exocytosis of miRNAs independently of exosomes in response to neuronal stimulation.
We propose a new function of non-coding RNAs named (‘ribomone’ = ribonucleotide + hormone), and suggest that miRNAs may function as hormones; i.e., miRNA is stored in vesicles and released by vesicle fusion in response to stimulation, thereby contributing to cell-to-cell communication.
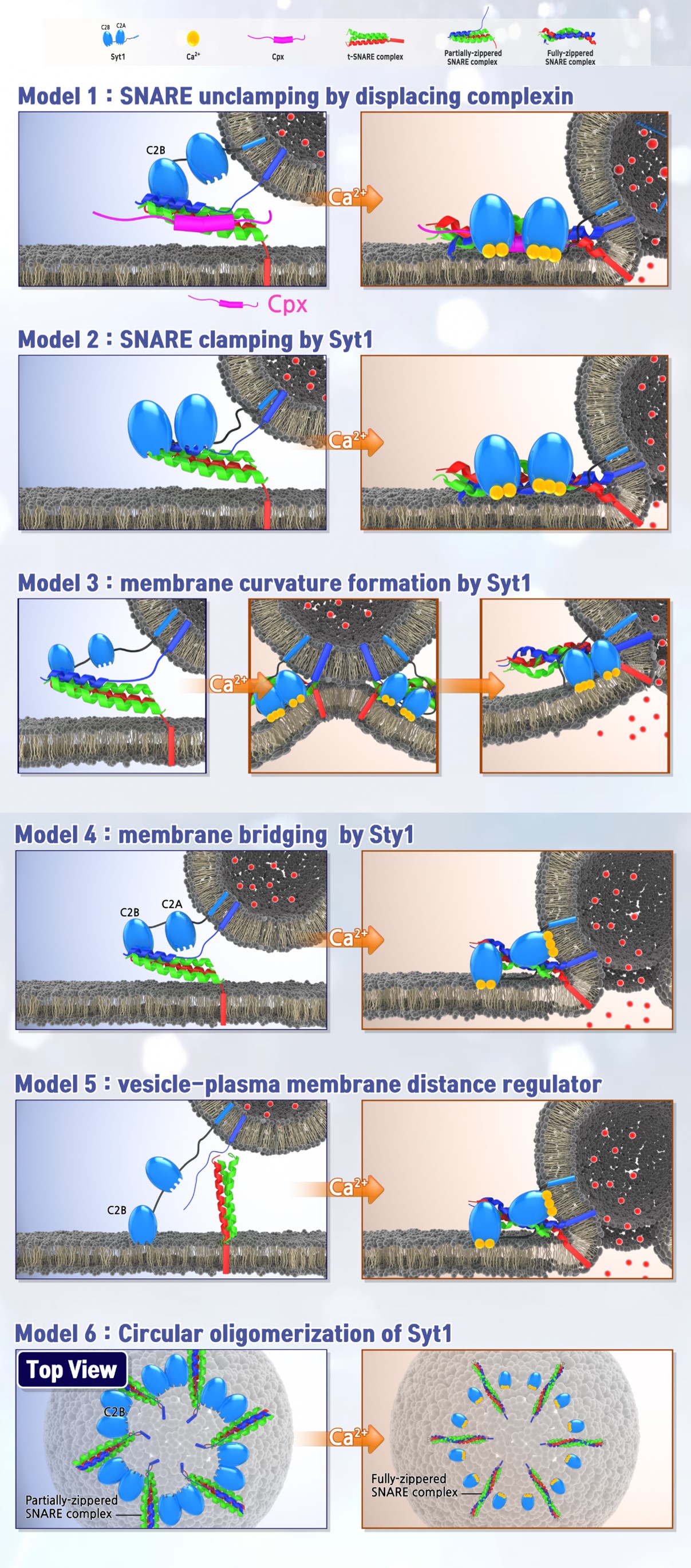
Lipid metabolism plays a crucial role in maintaining neuronal function, and alterations in lipid composition, including the reduction of phosphatidylinositol 4,5-bisphosphate (PIP2), have been implicated in neurodegenerative disorders. PIP2 is a key regulator of cell signaling and membrane trafficking, and its dysregulation is associated with neuronal dysfunction. Our research was the first to demonstrate that PIP2 functions as an electrostatic catalyst for vesicle fusion by lowering the hydration energy barrier (ACS Nano, 2024; Science Advances, 2025). We propose a paradigm shift in which PIP2 is recognized as a lipid catalyst for vesicle fusion, expanding our understanding of the molecular mechanisms governing neurotransmitter release.
Additionally, our findings indicate that cholesterol is essential for Ca²⁺-dependent vesicle fusion by enhancing membrane bending and deformation (Advanced Science, 2023). Using reconstitution assays with native vesicles, we identified a novel mechanism by which cholesterol facilitates vesicle fusion. Unlike synthetic liposomes, native vesicles are required for the reconstitution of physiological vesicle fusion. Our studies suggest that both PIP2 and cholesterol are essential for vesicle fusion, emphasizing that lipids can function as biological catalysts rather than passive structural components.
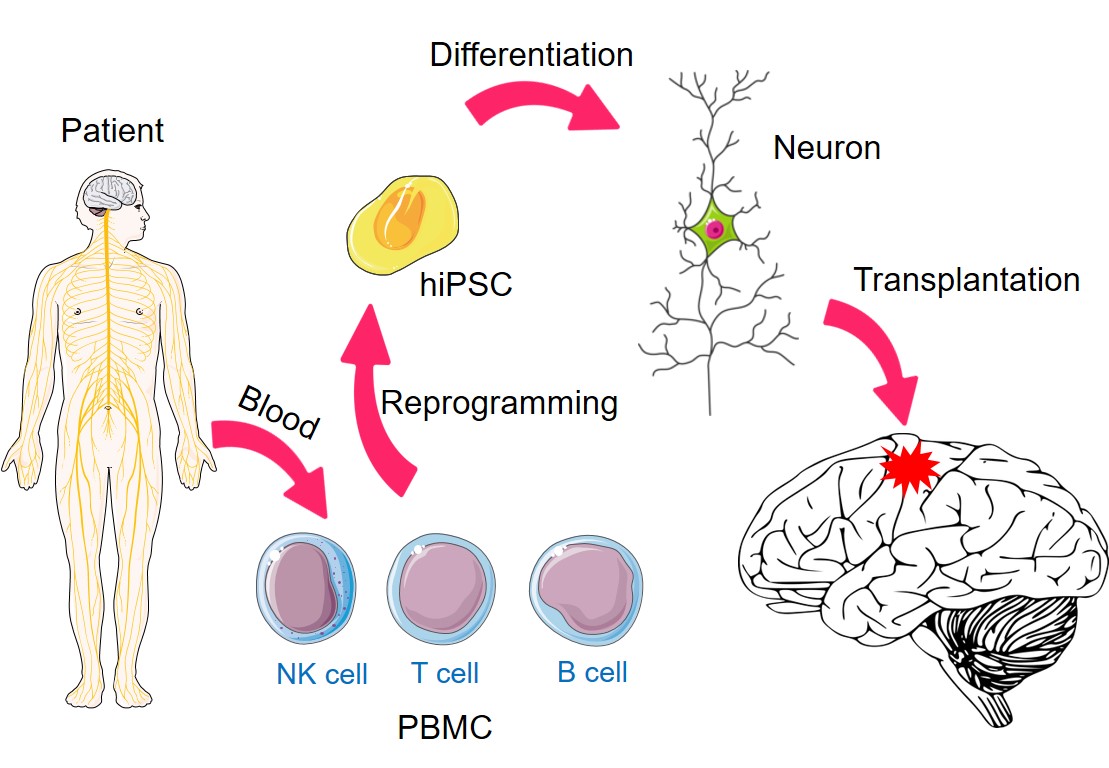
Autism spectrum disorder (ASD) is a complex neurodevelopmental condition characterized by social communication deficits and frequent comorbidities such as epilepsy. Genetic studies indicate that ASD-associated risk variants converge on calcium signaling pathways and vesicle fusion.
We employ functional assays such as single-cell calcium imaging, whole-cell patch-clamp electrophysiology, and microelectrode array (MEA) recordings to characterize these pathophysiological changes.